CITI Training
California State University, Chico and Chico State Enterprises has several training programs available for our research community. Some trainings are required for compliance purposes. Others are offered to help our research community gain a deeper knowledge of research and grants.
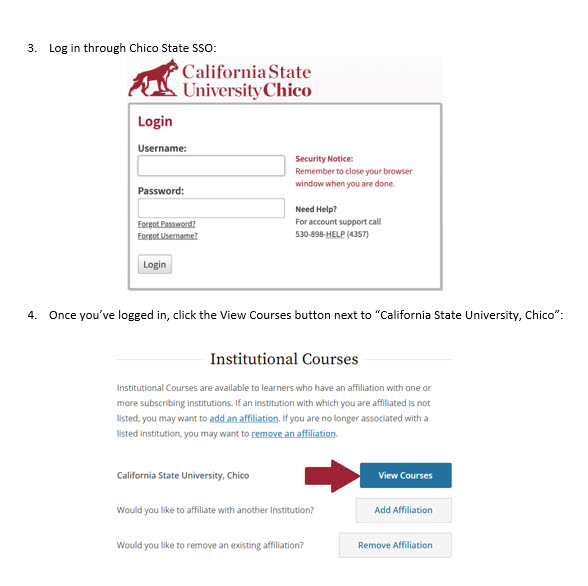
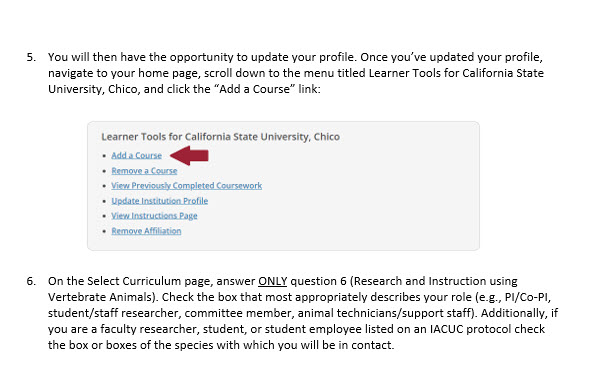
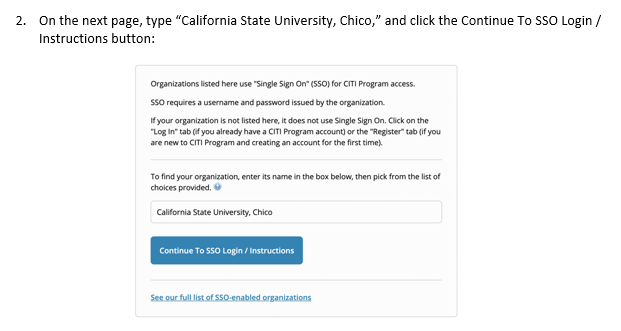
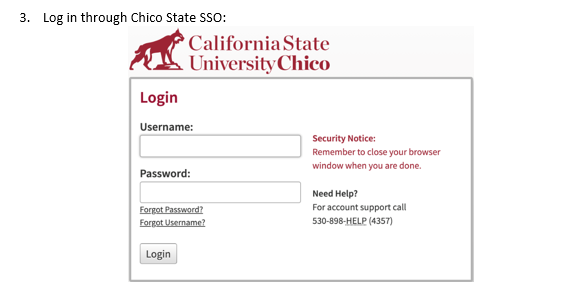
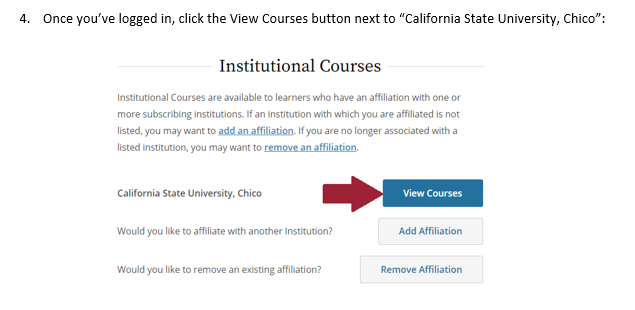
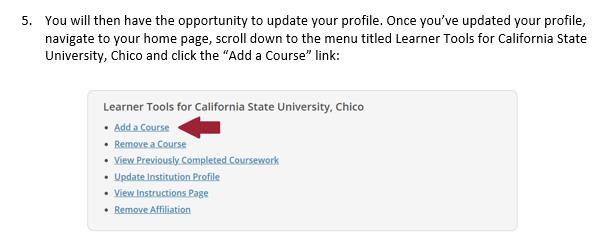
In the folders below are step-by-step registration instructions for creating an account and adding the appropriate training course to your account based on your role in a research project. Chico State has enabled the Single Sign-On (SSO) feature through CITI Training. Please use the "Log in Through My Institution" option when registering. Please use your Chico State email address when you complete your contact information during registration to allow integrations with other campus research programs like Cayuse.
Separate training courses are offered for each training series, such as Human Subjects, Animal Subjects, Biosafety, and Responsible Conduct of Research (RCR). Please read the different training series options carefully to ensure you enroll in and complete the appropriate training course for your role in the research project. If you complete the incorrect course, you must enroll in and complete the correct course.
Research ethics training certifications expire after three years. Upon expiration, committee members and individuals will automatically be enrolled in a refresher course. Individuals will be notified via email 90 days in advance of the expiry of each certification. If your research training requirement is related to an externally sponsored project, it is suggested that you complete the required training courses at the award notification stage to avoid delays in the project setup.
If you have questions about specific training modules, please contact Sharon Ruggirello at cititraininginfo@csuchico.edu.
The training courses are available online through CITI Program.
- Human Subjects Research
Who is required to complete ethics training?
Ethics training is required for all Key Personnel. Key Personnel is defined as:
1. Any Principal Investigator (PI), other investigators, and research personnel conducting human subjects that are (1) directly involved in conducting research (i.e., interacting and/or intervening) with human subjects; and/or (2) directly involved with handling private information and/or personally identifiable data related to human subjects during the course of a research project; or
2. Chico State faculty and staff who oversee undergraduate or graduate student PIs in their conduct of human subjects research; or
3. Collaborators from other institutions if Chico State serves as the IRB of record.
Site-based supervisors (e.g., cooperating teachers, master teachers, school psychologists, school counselors, etc.) who are supervising or mentoring a student PI who is completing a site-based research project, but who are not involved in data analysis are exempt from CITI training requirements. Similarly, individuals who may provide or work with de-identified data are exempt from CITI training as long as they have no knowledge of or access to identified data. Individuals not classified as Key Personnel (e.g., administrators, department chairs) may elect to complete relevant CITI training courses. PIs are ultimately responsible for ensuring that all Key Personnel on their studies have met the CITI training requirement.
What ethics training coursework is required?
Key Personnel is required to complete the appropriate training course. Individuals are required to self-identify which course to complete based on their role in the project. Once started, courses can be saved and completed at one’s convenience. Course completion times vary but may take between 1-2 hours to complete. In addition to the Required Modules, Supplemental Modules are available. Individuals are encouraged to identify Supplemental Modules that are relevant to their research. CITI training certificates remain active for 3 years, after which Key Personnel are required to renew their training via the appropriate Refresher course. Please note that additional CITI training (e.g., Responsible Conduct of Research) is required for Key Personnel affiliated with federally-sponsored projects or research funded by other agencies.
Accessing CITI Training for IRB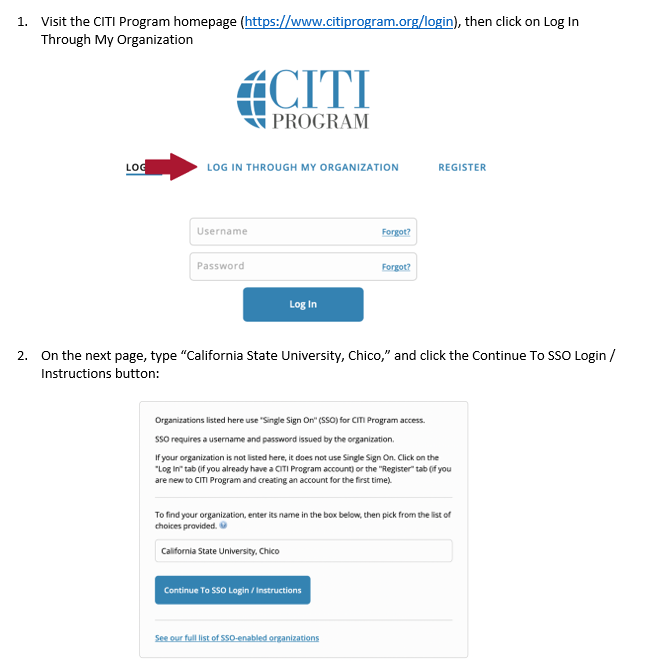
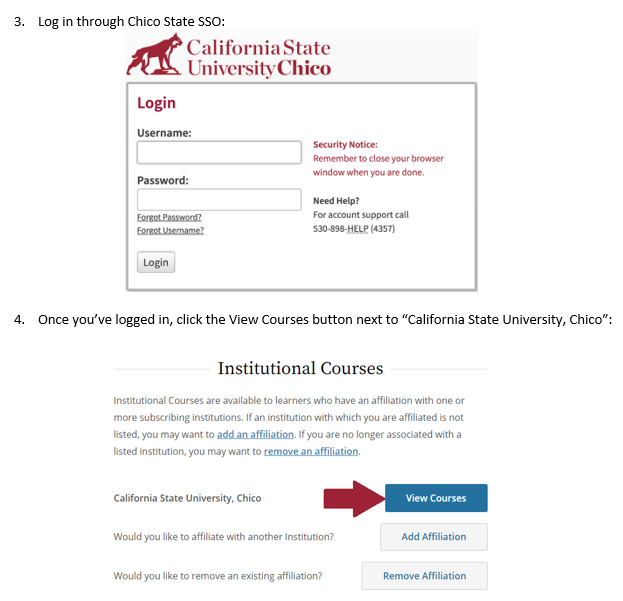
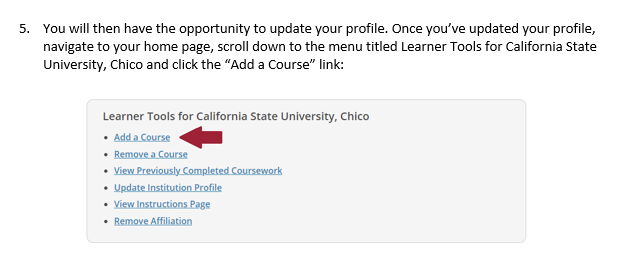
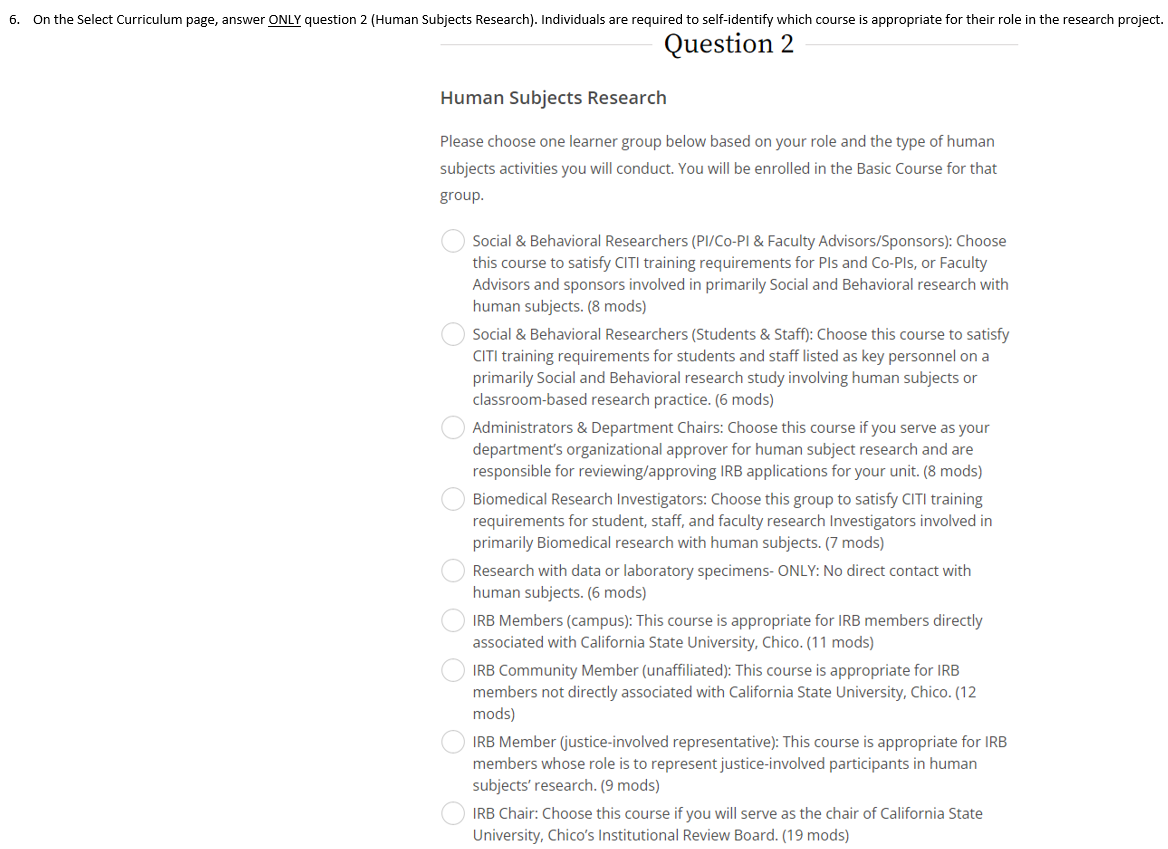

Accessing CITI Training for IRB (PDF) - Research and Instruction using Vertebrate Animals
Who is required to complete ethics training?
Ethics training is required for all Key Personnel. Key Personnel is defined as:
1. Any Principal Investigator (PI) as well as other investigators and research personnel that are directly involved in conducting research including interacting with, caring for, feeding, and handling animal subjects during the course of a research project.
2. Chico State faculty and staff who oversee undergraduate or graduate students in their conduct of animal subjects research.
3. Individuals not classified as Key Personnel (e.g., administrators, department chairs) may elect to complete relevant CITI training courses. Principal Investigators are ultimately responsible for ensuring that all Key Personnel on the study have met the CITI training requirements.
What ethics training coursework is required?
Key Personnel are required to complete the “Basic Training” course and species-specific elective modules to earn a completion certificate. This includes faculty researchers, student researchers, and student employees involved in or listed on a vertebrate animal research or instructional protocol. Individuals are required to self-identify which course to complete based on their role. Once started, courses can be saved and completed at one’s convenience. Course completion times vary but may take between 1-2 hours to complete. CITI training certificates remain active for 3 years, after which Key Personnel are required to renew their training via the appropriate Refresher course. Please note that additional CITI training (e.g., Responsible Conduct of Research) is required for Key Personnel affiliated with federally sponsored projects or research funded by other agencies.
Accessing CITI Training for IACUC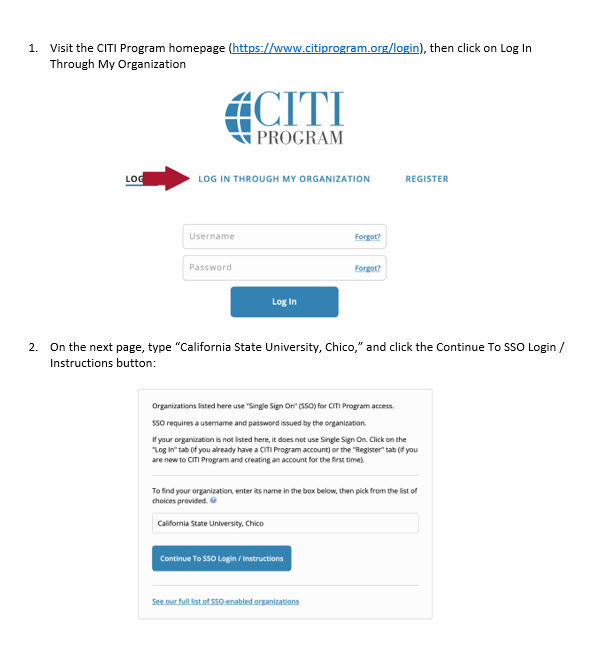


Accessing CITI Training for IACUC (PDF) - Biosafety and Security
Who is required to complete ethics training?
Biosafety training is required for all Key Personnel. Key Personnel is defined as:
1. Any Principal-Investigator (PI) or Co-Investigator (Co-PI), and all personnel including students and staff who are directly involved in or who handle biohazardous agents, including recombinant and/or synthetic nucleic acid molecules, listed on a protocol for Chico State IBC review and approval. 2. Chico State faculty and staff who oversee undergraduate or graduate students in their conduct of research or instructional research involving biohazardous agents or recombinant and/or synthetic nucleic acid molecules. 3. Individuals not classified as Key Personnel (e.g., administrators, department chairs) may elect to complete relevant CITI training courses. Principal Investigators are ultimately responsible for ensuring that all Key Personnel listed in the study have met the CITI training requirements.
What ethics training coursework is required?
Key Personnel are required to complete the “Initial” biosafety course to earn a completion certificate. This includes students, staff, and faculty involved in research, research training, and instructional activities using recombinant and synthetic nucleic acid molecules or other potentially hazardous biological agents. Individuals are required to self-identify which course to complete based on their role (e.g., PI/Co-PI or Students/Lab staff. Once started, courses can be saved and completed at one’s convenience. Course completion times vary but may take between 1-2 hours to complete. CITI training certificates remain active for 3 years. Upon expiration, a refresher course is required. Please note that additional CITI training (e.g., Responsible Conduct of Research) is required for Key Personnel affiliated with federally sponsored projects or research funded by other agencies.
Accessing CITI Training for IBC




- Responsible Conduct of Research
Training must be completed under the following circumstances:
Projects Funded by NSF
All undergraduate students, graduate students, and postdoctoral researchers supported by NSF funding, on a part-time or full-time basis, are required to complete RCR training. Training must be completed no more than ninety (90) days after a student, or postdoctoral researcher is hired to work on the project, and at least every four (4) years thereafter for the duration of the project.
Projects Funded by the National Institute of Food and Agriculture (NIFA), U.S. Department of Agriculture
All program directors, faculty, undergraduate students, graduate students, postdoctoral researchers, and any staff participating in a NIFA-funded research project are required to complete RCR training. Principal Investigators and project directors must complete the training within 90 days after acceptance of funding. Any others (students/staff/postdocs/etc.) must complete the training within 90 days after they first participate in the research. A refresher course is required at least every 4 years thereafter for the duration of the project.
Select NIH projects per solicitation/award guidelines
Live training is required for all undergraduate students, graduate students, and postdoctoral researchers supported by select NIH funding, on a part-time or full-time basis, are required to complete RCR training. Training must be completed no more than 90 days after a student, or postdoctoral researcher is hired to work on the project, and at least every 4 years thereafter for the duration of the project. The training plan must be provided to Chico State Enterprises (CSE). Since training will be live, the PI must provide appropriate evidence (agenda, sign-in-sheet, and/or certificates) to CSE. However, if the RCR management plan includes online CITI training, then students must complete training on the CITI website in addition to live training.
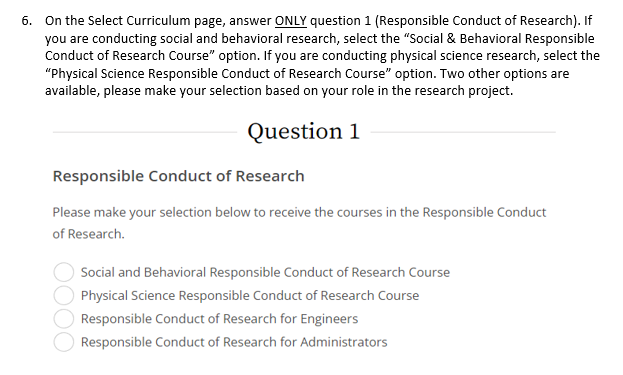
* Social and Behavioral Responsible Conduct of Research Course (basic/refresher)
* Physical Science Responsible Conduct of Research Course (basic/refresher)
* Responsible Conduct of Research for Engineers (basic/refresher)
* Responsible Conduct of Research for Administrators (basic/refresher)
Accessing CITI Training for RCR

Accessing CITI Training for RCR (PDF)
- Biosafety and Security
